Research Highlights : Investigate the microscopic mechamism of ion transport in iodide ionic liquid by first-principles calculations
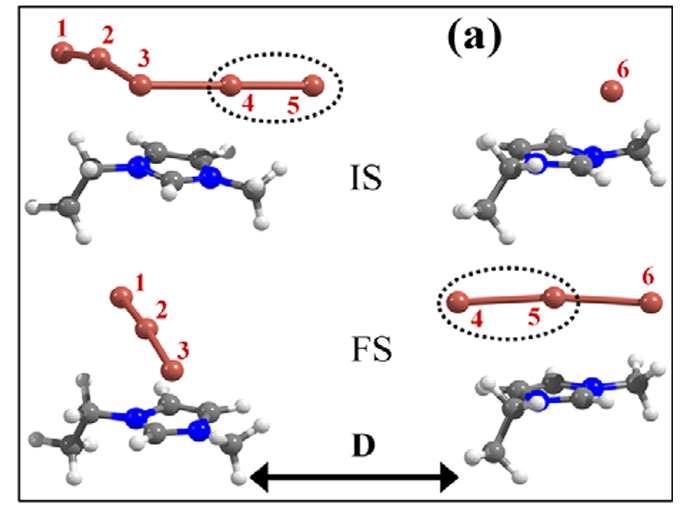
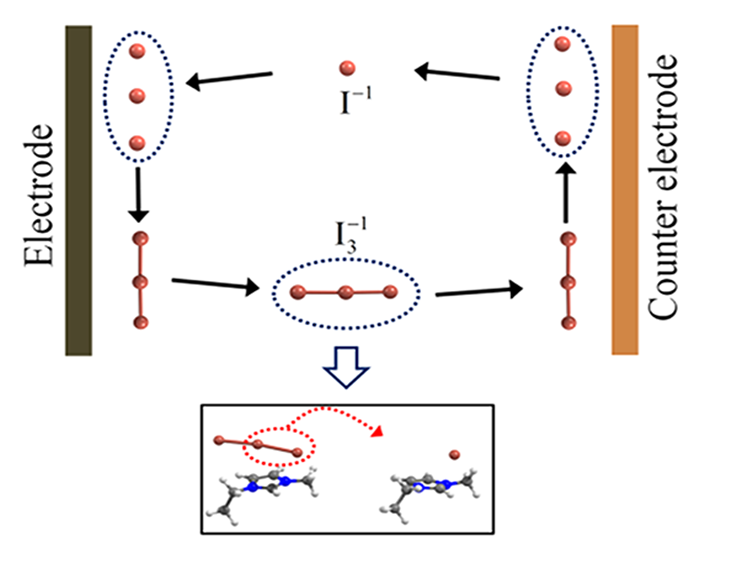
Abstract : We investigated the microscopic mechanism of ion transport in iodide ionic liquid, using first-principles calculations. We show that the desorption barrier of polyiodides (I3−or I5−) from the cation is in a similar energy range as or higher than the barrier for the bond dissociation and ensued desorption of neutral iodine (I2). This suggests that, instead of the physical diffusion of such a negatively charged multiatomic species, the exchange of neutral iodine (I2) between the polyiodides can be an easier channel for the movement of polyiodide. For the transport of the monoiodide anion (I−), we suggest the contribution of the Grotthuss-type ion exchange through the intermediately formed even-member anion (I2n−), in addition to drift and diffusion. As a result, we suggest that, instead of the commonly cited diffusion of the triiodide/iodide (I3−/I−) redox couple, the exchange of neutral iodine (I2) and the Grotthuss-type transport (I−) constitute the dominant ion transport mechanism
Resource : lion, leopard
Representative Thesis : Ranjit Thapa and Noejung Park* (2012) First-Principles Identification of Iodine Exchange Mechanism in Iodide Ionic Liquid. The Journal of Physical Chemistry Letters, 3, 3065-3069. corresponding author